上海典奥生物科技有限公司
14 年
手机商铺
商家活跃:
产品热度:
- NaN
- 0.9000000000000004
- 0.9000000000000004
- 1.9000000000000004
- 0.9000000000000004
推荐产品
公司新闻/正文
如何在1小时内精确定量检测COVID-19抗体浓度?
817 人阅读发布时间:2020-06-28 11:16
由SARS-CoV-2病毒导致的COVID-19大流行使全球范围内COVID-19疫苗、治疗方法、病毒感染和抗体反应诊断试验的开发迫在眉睫,因此也让研究者关注到需要更好的疫苗生物分析方法来跟地上这些快速发展的需求。
在研究对抗COVID-19大流行中,更大限度地提高免疫分析速度(获得结果的时间)、分析性能和重现性尤为重要,并且用于定量血清中COVID-19抗体的免疫分析、基于抗体的疗法的生物分析以及疫苗开发和生物处理过程中的生物分析,必须提高生产力和数据质量,并有助于满足大流行反应中积极的项目时间表。
作为专为生物治疗和疫苗开发领域研发生物分析技术的Gyros公司研发的Gyrolab SARS-CoV-2的抗体免疫分析技术,可以在1小时内精确定量COVID-19抗体,能够实现满足这些流行病相关免疫分析性能目标的解决方案, 为研究人员研究抗击COVID-19大流行提供帮助。
1. 技术优势
2. 检测原理
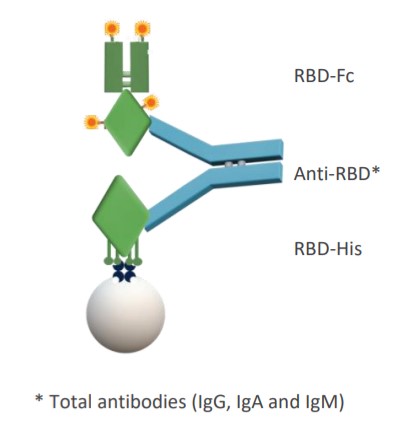
The three-step bridging Gyrolab SARS-CoV-2 antibody immunoassay utilizes a Gyrolab Bioaffy™ CD. The method encompasses a biotinylated SARS-CoV-2, Spike RBD-His recombinant protein as the capture molecule, and SARS‑CoV-2 Spike RBD-Fc recombinant protein labeled with Alexa Fluor® 647 as the detection molecule. This assay detects IgG, IgA and IgM antibody subtypes within the human serum.
在研究对抗COVID-19大流行中,更大限度地提高免疫分析速度(获得结果的时间)、分析性能和重现性尤为重要,并且用于定量血清中COVID-19抗体的免疫分析、基于抗体的疗法的生物分析以及疫苗开发和生物处理过程中的生物分析,必须提高生产力和数据质量,并有助于满足大流行反应中积极的项目时间表。
作为专为生物治疗和疫苗开发领域研发生物分析技术的Gyros公司研发的Gyrolab SARS-CoV-2的抗体免疫分析技术,可以在1小时内精确定量COVID-19抗体,能够实现满足这些流行病相关免疫分析性能目标的解决方案, 为研究人员研究抗击COVID-19大流行提供帮助。
Gyrolab SARS-CoV-2的抗体免疫分析技术
1. 技术优势
- 试剂消耗低:仅需1ug抗原蛋白,即可检测1千多个数据点。
- 样品体积小:4µL即可做重复
- 分析速度快:1CD只需要约1h(112个数据点)
- 检测范围广: ~40 ng/mL至~200µg /mL,可切换不同型号的CD,优化后获得更宽检测范围。
2. 检测原理

The three-step bridging Gyrolab SARS-CoV-2 antibody immunoassay utilizes a Gyrolab Bioaffy™ CD. The method encompasses a biotinylated SARS-CoV-2, Spike RBD-His recombinant protein as the capture molecule, and SARS‑CoV-2 Spike RBD-Fc recombinant protein labeled with Alexa Fluor® 647 as the detection molecule. This assay detects IgG, IgA and IgM antibody subtypes within the human serum.
3. 分析范围
Gyrolab SARS-CoV-2的抗体免疫分析技术检测动态范围为40 ng/mL——200μg/mL。可切换不同型号的CD,优化后获得更宽检测范围。
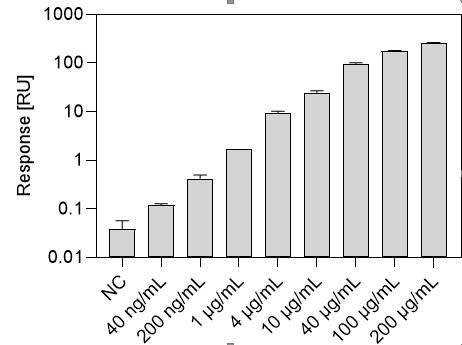
Average responses obtained for a negative control (NC) and positive controls ranging from 40 ng/mL to 200 µg/mL (in neat serum) in Rexxip H containing 25% human serum. Rabbit polyclonal anti-SARS-CoV-2 Spike RBD antibody was used as positive control.
Gyrolab 纳升级高通量微流控免疫分析工作站
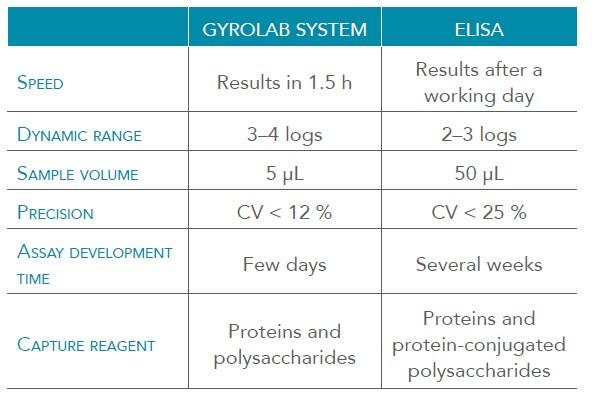
Gyrolab SARS-CoV-2的抗体免疫分析技术依托于Gyrolab 纳升级高通量微流控免疫分析工作站,该平台可以提供时间关键工作流程所需的性能,包括快速开发方法、分析样品、处理小样本和节省试剂体积,以及在大动态范围内以最小基质干扰进行测量的能力,被认为是加速生物制药发展和生产的必备技术。

点击图片观看Gyrolab视频介绍
Gyrolab是一种强大的免疫分析平台,将传统的抗体抗原免疫大分子检测方法与专利的微流控CD技术完美结合,能够做到其他需要人工操作的实验方法所达不到的实验效果。
1. Gyrolab优势:
- 微量化 :微流控技术、纳升级上样、最小上样量20nL
- 速度快 :1小时内完成1片CD的样品分析(上样、清洗、数据处理)
- 通量高 :每片CD112个数据点,每批次可做5片CD,即560个数据点
- 基质效应低 :适用于任何样品基质 MRD大幅降低,可达2倍
- 自动化 :避免人为误差,降低CV值 可以过夜实验,提高通量
- 检测范围广:拓宽LLOQ和ULOQ,完美覆盖不同浓度范围的样品
2. Gyrolab在疫苗开发中的应用
疫苗开发过程中的生物分析在分析复杂性、样品基质、分析物类型的多样性以及对工作流程效率的要求等方面提出了许多挑战。由于COVID-19疫苗开发的项目时间表经常被压缩以缩短开发时间,从而达到抗击流行病的目的,因此对快速生成数据的需求也越来越大。Gyrolab平台因为消除了手动ELISA长时间孵化时间,能够更大限度地提高免疫分析速度。
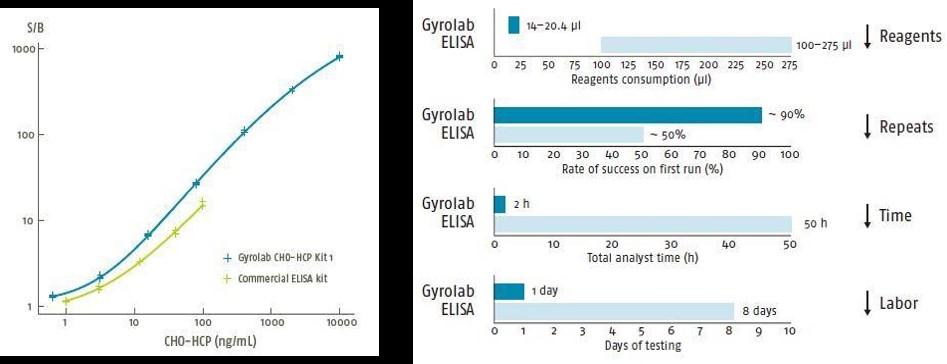
Customer example of improvements in analysis time, dynamic range, sample savings, and assay development time when transitioning from ELISA to Gyrolab immunoassay.
Gyrolab平台免疫分析用于疫苗开发,以表征疫苗的滴度、纯度、亲和力和效力,以及它们在动物和人类中的免疫原性反应。Gyrolab分析灵活性允许在单个Gyrolab Bioaffy CD上运行多个分析,如下例所示。
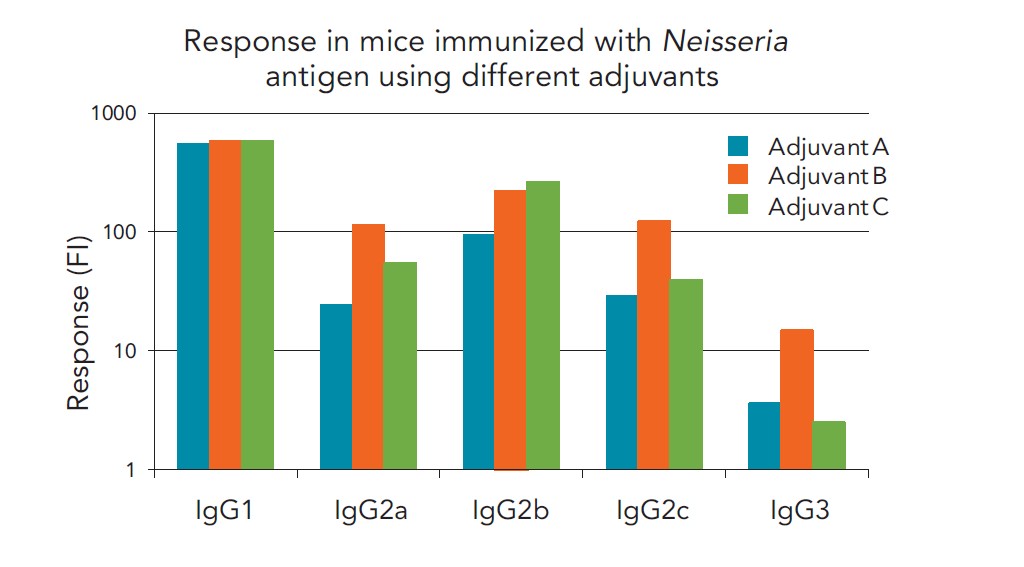
Immunogenicity responses in a preclinical mouse study detecting IgG antibody subtypes simultaneously in a single run were analyzed with Gyrolab immunoassays to screen responses from different formulations.
点击图片观看Gyrolab 在COVID-19中的应用详情(webinar视频)
3. Gyrolab 其他应用领域
Gyrolab免疫实验平台能够很好地满足您在生物大分子药物研发、临床前、临床、生产等各环节的免疫学实验需求。
- PK/TK(药/毒代动力学)
- ADA(抗药抗体、免疫原性)
- biomarker(生物标记物)
- PD(药效动力学)
- bioprocess(HCP、Protein A、P24、AAV、IgG titer)
- 亲和力检测等生物分析
目前,Gyrolab被广泛应用于众多生物制药、CRO、CMO、高校和研究所,如需了解更多,欢迎联络我们。






